Kinetic Energy
Kinetic energy is the energy of motion. An object which has any type of motion has kinetic energy. There are many forms of kinetic energy:
- Electron: the energy due to the motion of electrons around the nucleus of an atom
- Vibrational: the energy due to vibrational motion typical of atoms/molecules within solids, liquids and gases (notice that there mary ways in which the springs (imaginary intermolecular connections) can compress and stretch; hence, vibrate)
- Rotational: the energy due to rotational motion typical of more energetic states of liquids and gases
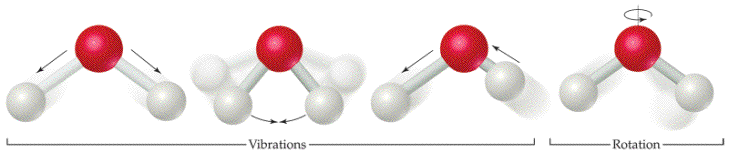
- Translational: the energy due to motion from one location to another typical of the highest energy states such as gases
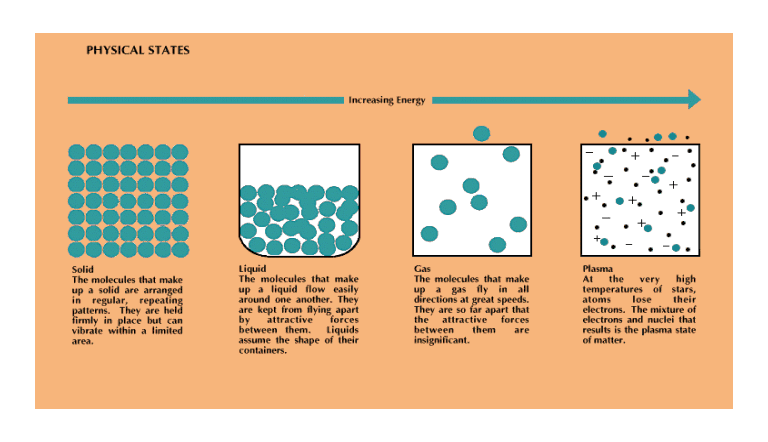
This unit is about the behaviour of gases so this is where the focus will remain. This is because gases are easiest to study because they move with little energy loss and are simple to manipulate. This was why they were used to develop much of the chemistry we understand and rely on today. Gases are characterized by possessing translation kinetic energy. Translational kinetic energy depends upon two variables: the mass (m) of the object and the speed (v) of the object. The following equation is used to represent the kinetic energy (KE) of an object.
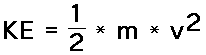
This equation reveals that the kinetic energy of an object is directly proportional to the square of its speed. That means that for a twofold increase in speed, the kinetic energy will increase by a factor of four. For a threefold increase in speed, the kinetic energy will increase by a factor of nine. Kinetic energy is a scalar quantity; it does not have a direction and is completely described by magnitude alone. Like work and potential energy, the standard metric unit of measurement for kinetic energy is the Joule. As might be implied by the above equation, 1 Joule is equivalent to 1 kg*(m/s)2.
Temperature
The Fahrenheit scale (°F) was the first widely used temperature scale. It was developed in the early 18th century by G. Daniel Fahrenheit. The zero point of the Fahrenheit scale is attained by mixing equal parts of water, ice, and salt. Fahrenheit set the number 32 at the freezing point of water. He set the boiling point of water to 212 on his scale. 180 equal divisions or degrees (°) are marked on the Fahrenheit scale between the freezing and boiling points of water. The Celsius temperature scale (°C) was developed by Anders Celsius in 1742. The zero point of the Celsius scale is set to the temperature at which water freezes. The number 100 is set to the temperature at which water boils. Celsius set 100 equal divisions (degrees) between the freezing and boiling points. The Kelvin temperature scale (K) was developed by Lord Kelvin in the mid 1800s. Lord Kelvin developed this scale with the help of a Carnot engine. The Carnot engine deals with the relationship between pressure, work, and temperature. The Carnot engine is the most efficient engine possible. However, even it cannot reach 100% efficiency. If an engine was 100% efficient, no energy would be wasted. The Carnot engine is only theoretical, which means that all real engines are even less efficient.

The zero point on this scale is base on the point at which the pressure of all dilute gases mathematically project to zero from the triple point of water; the temperature at which liquid water, ice, and water vapor can coexist simultaneously. The zero point of this scale is equivalent to -273.16 °C on the Celsius scale. This zero point is considered the lowest possible temperature of anything in the universe. Therefore, the Kelvin scale is also known as the absolute temperature scale. At this temperature, the gas has no volume or pressure and the molecules have absolutely no energy (no pun intended). Scientists believe that this point can never be reached because it would be impossible to eliminate electron kinetic energy due to repulsion forces within an atom. At the freezing point of water, the temperature of the Kelvin scale reads 273 K. At the boiling point of water, it reads 373 K.
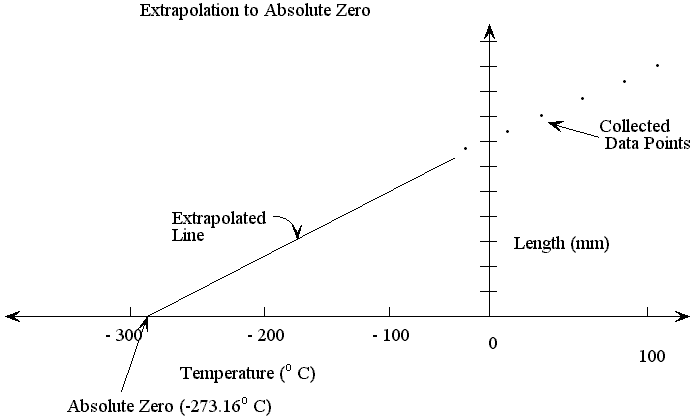
To convert between Kelvins and degrees Celsius, the following formula is used: 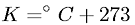 . The Kelvin scale is preferred because it contains no negative numbers and is not arbitrary in that it truly reflects the energy state of a system. Thus, it is the only real measurement of temperature which reflects the kinetic energy of something. Fahrenheit and Celsius are really relative measures of hotness.
. The Kelvin scale is preferred because it contains no negative numbers and is not arbitrary in that it truly reflects the energy state of a system. Thus, it is the only real measurement of temperature which reflects the kinetic energy of something. Fahrenheit and Celsius are really relative measures of hotness.
Pressure
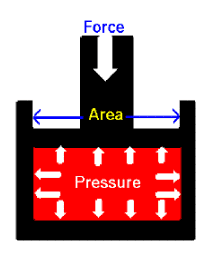
Gases are often defined by their pressure. Everyone has some understanding of pressure because we here the term a lot and we have felt the pressure created when we fill something like a balloon with gas. However, what is pressure really? We know that a gas is composed of a large number of molecules that are very small relative to the distance between molecules. These gas molecules are in constant, random motion and frequently collide with each other and with the walls of any container. When the gas molecules collide with the container, we assume that no energy is transferred to the container, that is, the collision is perfectly elastic. However, when the molecules hit the container they do impart a perpendicular force on that wall. Gas pressure is generated by the continual bombardment of a tremendous number of perfectly elastic collisions of gas particles on a surface. This is a force extended of a unit area, or force/area or N/m2. The S.I. unit is a pascal (Pa). Like Kinetic energy, pressure is a scalar quantity; it does not have a direction and is completely described by magnitude alone.
Traditionally gas pressure was measured by the extent to which the gas pushed mercury up a small tube.
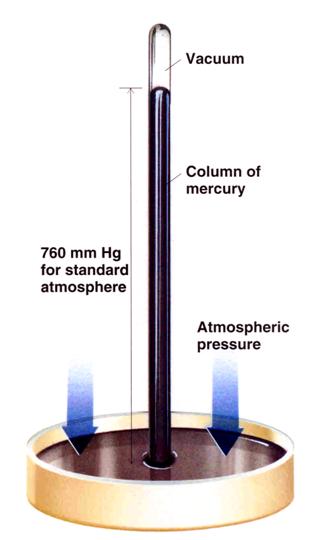
This unit of mm of mercury has been replaced with many others including bar, atmospheres and etcetera, but pascals is metric. 760 mm of mercury is the height to which air can push mercury up a tube at 273 K. This pressure is also 101.3 kPa.
Gas Laws
There are several key relationships which can used to determine pressure, temperature, volume or moles of gas present. Many of these formulas led the way to modern chemistry.

The first of the gas law studies was conducted by Robert Boyle in 1662. He used a J-shaped piece of glass tubing that was sealed on one end. A gas (air) was trapped in the sealed end of the tube and varying amounts of mercury were added to the J-shaped tube to vary the pressure of the system. Boyle systematically varied the pressure and measured the volume of the gas. These measurements were performed using a fixed amount of gas and a constant temperature. In this way Boyle was able to examine the pressure-volume relationship without complications from other factors such as changes in temperature or amount of gas.
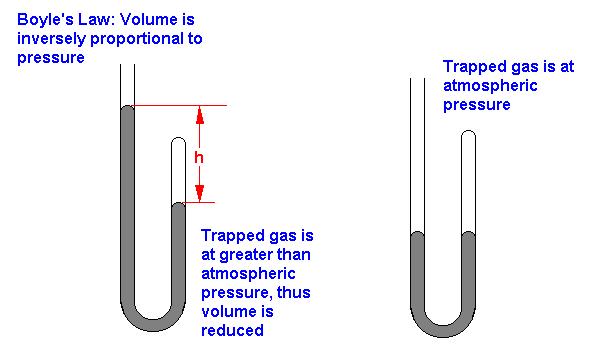
Thus, the volume of a fixed amount of gas at constant temperature is inversely proportional to the pressure. If the volume of the container of gas was suddenly increased, the gas molecules would have further to travel to collide with the walls of the container and hence the pressure would decrease (less collisions per second). This inverse relationship is best depicted as:
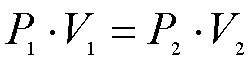
E.g. If 50 mL of oxygen gas is compressed from 20 atm of pressure to 40 atm of pressure, what is the new volume at constant temperature?
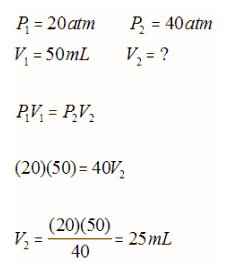
In an effort to make hot air balloons fly better during the early 1800's in France, Jacques Charles and Joseph-Louis Gay-Lussac, made detailed measurements on how the volume of a gas was affected by the temperature of the gas.

Jacques Charles took care to keep all properties of the gas constant except for temperature and volume. His experiments involved trapping a small quantity of gas in a glass tube that was sealed at one end. This tube was immersed in a water bath and by changing the temperature of the water, Charles was able to change the temperature of the gas. The pressure was held constant by keeping the other end of the tube open and thus the pressure was always equal to the atmospheric pressure.
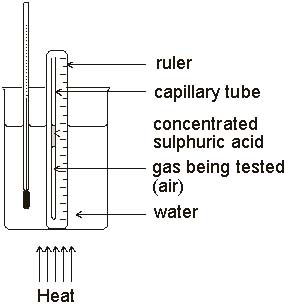
Jacques Charles noted that the volume of a gas increased with the temperature. Charles's Law states that the volume of a given amount of dry ideal gas is directly proportional to the Kelvin Temperature provided the amount of gas and the pressure remain fixed. If the temperature of a gas is increased, the gas molecules would be moving faster resulting in more collisions per second on the wall of the container and each collision would have more force both of which result in an increase in pressure. Thus the relationship between volume and temperature (must be in Kelvins) of a gas can be stated as:
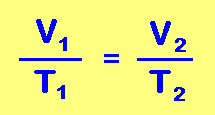
E.g. A sample of gas at 101.3kPa had a volume of 1.2 L at 100oC. What would its volume be at 0oC at the same pressure?
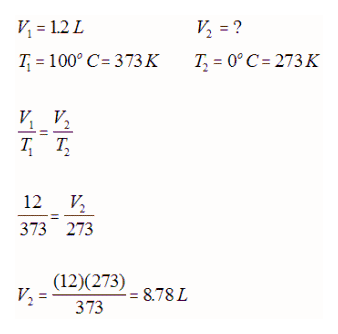
If you allow temperature, pressure and volume to change but keep the type and amount of gas constant, you are combining these gas laws into one equation which can be represented by:
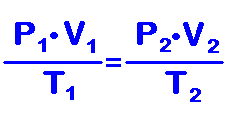
E.g. A 320 cm3 sample of helium gas is at STP. What was the temperature if the conditions if the volume was changed to 350 cm3at a pressure of 99.3 kPa?
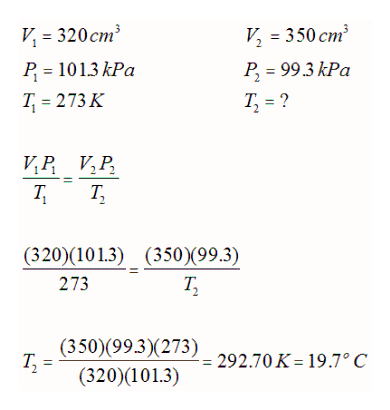
John Dalton (1766–1844) was born in Cumberland, England, and earned his living for most of his life as a teacher and public lecturer, beginning in his village school at the age of 12.

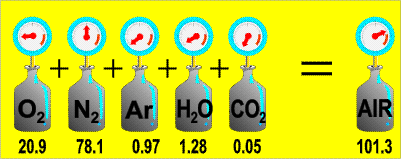
He studied the effect of gases in a mixture. He observed that the Total Pressure of a gas mixture (non-reacting) was the sum of the Partial Pressure of each gas.
Since there was an enormous amount of space between the gas molecules within the mixture that the gas molecules did not have any influence on the motion of other gas molecules, therefore the pressure of a gas sample would be the same whether it was the only gas in the container or if it were among other gases. This assumption that molecules act independently of one another works fine as long as there is a lot of space between gas molecules in the mixture and the temperature is not too low.
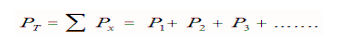
Typically, total pressure is known, but a partial pressure is not. The equation used to determine the partial pressure of any mixture of non-reacting gases is simply the mole fraction for that gas (moles of that gas/total moles of gas) multiplied by the total pressure or:
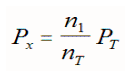
Determine the partial pressure of of H2 in a mixture made up of 6 grams of H2, and 32 grams O2 if the total pressure is 750 torr?
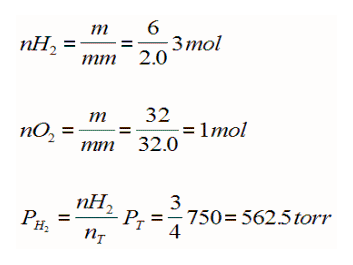
These gas laws are true as long as the gases do not react with each other and all collisions are perfectly elastic. This represents an ideal condition, but in reality gases only approximate these conditions as long as the pressure is moderate and the temperature is well above the condensation point for that gas.
The last change to consider deals with the amount of gas. However, the first question is, how does the amount of gas relate to the conditions of that gas. An ideal gas can be characterized by three state variables: pressure (P), volume (V), and Kelvin temperature (T). The relationship between them may be deduced from kinetic theory and is called the ideal gas law. The equation is:
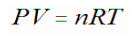
In this equation n represents moles and R is the ideal gas law constant:
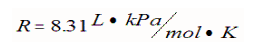
E.g. How many moles of gas is required to generate a pressure of 104 kPa at 18oC if the volume of the container is 3.1 L?
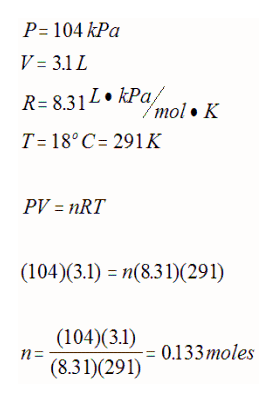
If you use this equation, you can calculate the volume of 1 mole of gas at STP (22.4 L) or at SATP (24.8 L). The volume of any gas at these standard conditions is called the molar volume of a gas. This can be used to stoichiometrically determine moles or volume as long as standard conditions are observed.
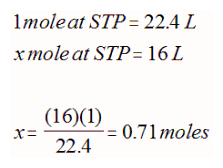
Since we know have 2 new ways to determine moles (ideal gas law and molar volume), stoichiometric calculations can be extended to include gases. For example, if 500 mL of HCl gas at 300 K and 100 kPa dissolved in pure water requires 12.50 mL of the NaOH solution to neutralize in a titration experiment, what is the concentration of the NaOH solution?
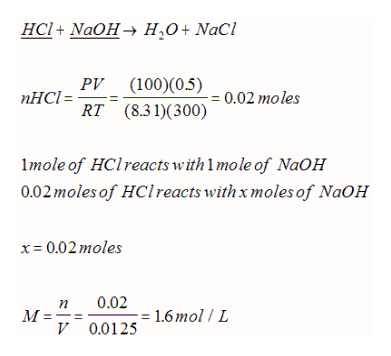
Recall Avogadro's hypothesis stated that equal volumes of gases at the same temperature and pressure must contain the same number of gas particles. This can be used to simplify gas stoichiometry if the gases investigated are measured under identical conditions. However, this gas only be used to compare two gases within a reaction (it does not matter if there are other solids or solutions or liquids within the reaction). For example: If 3.5 L of H2 gas reacts with ample N2 gas, what volume of NH3 gas can be collected if all the gases are measured at the same 85 kPa and 23oC?
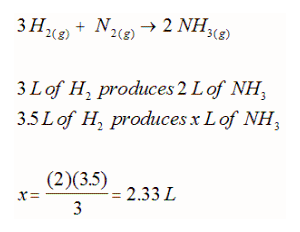
References
http://www.ilpi.com/msds/ref/concentration.html
http://www.iupac.org/goldbook/M03722.pdf
http://www.cdc.gov/Niosh/idlh/74908.html
http://www.lenntech.com/hazardous-substances/acrylonitrile.htm
http://www.lakes-environmental.com/toxic/TETRACHLOROETHYLENE.HTML
http://www.sciencelab.com/xMSDS-Page_8_Solution-9926370
http://www.hc-sc.gc.ca/fn-an/securit/chem-chim/acrylamide/acrylamide_level-acrylamide_niveau_e.html
http://www.buhs.k12.vt.us/science/physicalscience/Lab%20images/0009-004-sol-v-t.gif
http://www.thesciencedesk.com/sgsolubilitygraph.htm
http://patrick.wattle.id.au/cameron/cshs/hammond/weekend/ppt2.jpeg
http://dl.clackamas.edu/ch105-04/molarity.htm
http://misterguch.brinkster.net/molaritytutorial.html
http://dl.clackamas.edu/ch105-04/calculat.htm
http://dl.clackamas.edu/ch105-04/dilution.htm
http://www.iun.edu/~cpanhd/C101webnotes/aqueoussolns/solstoic.html
http://www.chemistry.mtu.edu/pages/courses/files/ch1120-sgreen/chap19_lec_sg_files/slide0016_image016.gif
http://nsr.mij.mrs.org/9/4/figure6.gif
http://www.glenbrook.k12.il.us/GBSSCI/PHYS/Class/energy/u5l1c.html
http://sol.sci.uop.edu/~jfalward/thermodynamics/atomsonsprings.jpg
http://upload.wikimedia.org/wikipedia/en/thumb/0/00/Gas-particles.jpg/250px-Gas-particles.jpg
http://cache.eb.com/eb/image?id=63065&rendTypeId=4
http://www.windows.ucar.edu/earth/Atmosphere/temperature/celsius.html
http://www.windows.ucar.edu/earth/Atmosphere/temperature/fahrenheit.html
http://www.windows.ucar.edu/earth/Atmosphere/temperature/kelvin.html
http://www.sciencebyjones.com/zero_extrapolated.gif
http://www.equationsheet.com/png/0382.png
http://www.grc.nasa.gov/WWW/K-12/airplane/pressure.html
http://wright.nasa.gov/airplane/pressure.html
http://img.sparknotes.com/content/testprep/bookimgs/sat2/chemistry/0003/sat117002_0508.gif
http://members.aol.com/profchm/boyle.html
http://www.subrosa.com.tr/internet/alcimizm/alcimizm_clip_image008_0001.jpg
http://neon.chem.uidaho.edu/~honors/boyle.jpg
http://www.fargo.k12.nd.us/education/components/scrapbook/default.php?sectiondetailid=8173&sc_timestamp=1116538237
http://members.aol.com/profchm/charles.html
http://www.chm.davidson.edu/ChemistryApplets/GasLaws/CharlesLaw.html
http://www.saburchill.com/physics/images6/041203001.jpg
http://www.1728.com/charles.htm
http://www.1728.com/combined.htm
http://www.chemheritage.org/classroom/chemach/periodic/dalton.html
http://members.aol.com/profchm/dalton.html
http://www.space.gc.ca/asc/eng/educators/resources/neemo/gases/partial.asp
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html
http://www.science.uwaterloo.ca/~cchieh/cact/c120/gastoichiometry.html

