Defining Equilibrium
Basically, the term refers to what we might call
a "balance of forces". In the case of mechanical equilibrium,
this is its literal definition. A book sitting on a table top remains at
rest because the downward force exerted by the earth's gravity acting on
the book's mass (this is what is meant by the "weight" of the book) is
exactly balanced by the repulsive force between atoms that prevents two
objects from simultaneously occupying the same space, acting in this case
between the table surface and the book. If you pick up the book and raise
it above the table top, the additional upward force exerted by your arm
destroys the state of equilibrium as the book moves upward. If you wish
to hold the book at rest above the table, you adjust the upward force to
exactly balance the weight of the book,
thus restoring equilibrium. An object is in a state of mechanical
equilibrium when it is either static (motionless) or in a state of unchanging
motion. From the relation f = ma , it is apparent that if the net
force on the object is zero, its acceleration must also be zero, so if
we can see that an object is not undergoing a change in its motion, we
know that it is in mechanical equilibrium.

Consider a person on a bungee cord. When the
jump off a bridge they are not in mechanical equilibrium since the are
rapidly moving towards the Earth due to gravity. As the bungee cord
extends, they reach equilibrium for an instant when the force of gravity
equals the opposing force of the bungee cord retracting and the person
stops moving. However, as soon as the bungee cord retracts and the
person accelerates upward, equilibrium is lost until the forces again equal
at the top. This cycle continues until the force causing the bungee
cord to recoil is gone. At which point the equilibrium is permanent (more
or less) and the person stops bobbing up and down (more or less).
Another kind of equilibrium we all experience is
thermal
equilibrium. When two objects are brought into contact, heat will flow
from the warmer object to the cooler one until their temperatures become
identical. Thermal equilibrium is a "balance of forces" in the sense that
temperature is a measure of the tendency of an object to lose thermal energy.
A metallic object at room temperature will feel cool to your hand when
you first pick it up because the thermal sensors in your skin detect a
flow of heat from your hand into the metal, but as the metal approaches
the temperature of your hand, this sensation diminishes. The time it takes
to achieve thermal equilibrium depends on how readily heat is conducted
within and between the objects; thus a wooden object will feel warmer than
a metallic object even if both are at room temperature because wood is
a relatively poor thermal conductor and will therefore remove heat from
your hand more slowly. Thermal equilibrium is something we often want to
avoid, or at least postpone; this is why we insulate buildings, perspire
in the summer and wear heavier clothing in the winter.
Many reactions in chemistry go 100% to completion
such as burning methane or simple acid/base neutralizations. However,
many reactions are complete when they are in fact only partially converted
to products. These reactions exist in a dynamic state of flux in
which reactants become products and products become reactants. Previously
we would have defined these reactions as incomplete and described the factors
reduced the percentage yield. In actuality, these reactions are completely
done reacting but they are not 100% complete. These reactions are
reversible and exist in a state of dynamic equilibrium.
There are several types of equilibria that a chemist
could look at. There is phase equilbria in which a
substance constantly fluctuates between two states such as water vapour
and condensation. There is solubility equilbria in
which a solid is constantly dissolving and precipitating. There is
redox
equilibria in which ions are constantly gaining and losing electrons
to exist between two states such as Fe2+ and Fe3+.
For the most part chemists deal with reaction equilibria in which
the amount of reactant and products are constantly reacting back and forth
regenerating each other.
When a chemical reaction takes place in a container
which prevents the entry or escape of any of the substances involved in
the reaction, the quantities of these components change as some are consumed
and others are formed. Eventually this change will come to an end,
after which the composition will remain unchanged as long as the system
remains undisturbed. The system is then said to be in its equilibrium state,
or more simply, "at equilibrium". A chemical reaction is in equilibrium
when there is no tendency for the quantities of reactants and products
to change. The direction in which we write a chemical reaction (and
thus which components are considered reactants and which are products)
is arbitrary because the reaction is proceeding at equal rates in both
directions. However, this does not imply that the amount of reactants
and products are equal, just that they are no longer changing.
Thus the two equations:
H2 + I2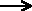 2 HI "synthesis of hydrogen iodide"
2 HI "synthesis of hydrogen iodide"
2 HI  H2
+ I2 "dissociation of hydrogen iodide"
H2
+ I2 "dissociation of hydrogen iodide"
represent the same chemical reaction system in which the roles of the
components are reversed, and both yield the same mixture of components
when the change is completed. This last point is central to the concept
of chemical equilibrium. It makes no difference whether we start with two
moles of HI or one mole each of H2 and I2; once the
reaction has run to completion, the quantities of these two components
will be the same. In general, then, we can say that the composition of
a chemical reaction system will tend to change in a direction that brings
it closer to its equilibrium composition. Once this equilibrium composition
has been attained, no further change in the quantities of the components
will occur as long as the system remains undisturbed. Plotting data
for these two reactions would then yield the same results:
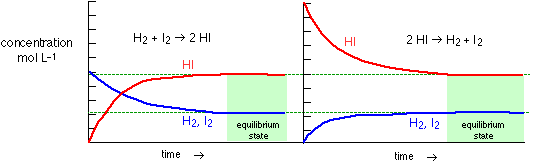
Thus a chemical equation of the form A  B represents the transformation of A into B, but it does not imply that
all of the reactants will be converted into products, or that the reverse
reaction B
B represents the transformation of A into B, but it does not imply that
all of the reactants will be converted into products, or that the reverse
reaction B  A cannot
also occur. In general, both processes can be expected to occur,
resulting in an equilibrium mixture containing finite amounts of all of
the components of the reaction system. (We use the word components when
we do not wish to distinguish between reactants and products.) If
the equilibrium state is one in which significant quantities of both reactants
and products are present (as in the hydrogen iodide example given above),
then the reaction is said to incomplete or reversible, but the latter term
is preferable because it avoids confusion with "complete" in its other
sense of being finished, implying that the reaction has run its course
and is now at equilibrium.
A cannot
also occur. In general, both processes can be expected to occur,
resulting in an equilibrium mixture containing finite amounts of all of
the components of the reaction system. (We use the word components when
we do not wish to distinguish between reactants and products.) If
the equilibrium state is one in which significant quantities of both reactants
and products are present (as in the hydrogen iodide example given above),
then the reaction is said to incomplete or reversible, but the latter term
is preferable because it avoids confusion with "complete" in its other
sense of being finished, implying that the reaction has run its course
and is now at equilibrium.
If it is desired to emphasize the reversibility of
a reaction, the single arrow in the equation is replaced with a pair of
hooked lines pointing in opposite directions, as in A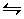 B.
However, there is no fundamental difference between the meanings of A
B.
However, there is no fundamental difference between the meanings of A  B and A
B and A B.
A reaction is said to be complete when the equilibrium composition contains
no significant amount of the reactants. However, a reaction that is complete
when written in one direction is said "not to occur" when written in the
reverse direction. In principle, all chemical reactions are reversible,
but this reversibility may not be observable if the fraction of products
in the equilibrium mixture is very small, or if the reverse reaction is
kinetically inhibited (very slow.)
B.
A reaction is said to be complete when the equilibrium composition contains
no significant amount of the reactants. However, a reaction that is complete
when written in one direction is said "not to occur" when written in the
reverse direction. In principle, all chemical reactions are reversible,
but this reversibility may not be observable if the fraction of products
in the equilibrium mixture is very small, or if the reverse reaction is
kinetically inhibited (very slow.)
Chemical equilibrium and reaction reversibility was
confirmed by many chemists, but most notably by two Norwegian chemists
(and brothers-in-law) Cato Guldberg and Peter Waage. During the period
1864-1879 they showed that an equilibrium can be approached from either
direction, implying that any reaction aA + bB cC + dD is really a
competition between a "forward" and a "reverse" reaction. When a reaction
is at equilibrium, the rates of these two reactions are identical, so no
net (macroscopic) change is observed, although individual components are
actively being transformed at the microscopic level. Guldberg and
Waage showed that the rate of the reaction in either direction is proportional
to what they called the "active masses" of the various components:
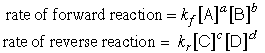
in which the proportionality constants k are called rate constants and
the quantities in square brackets represent concentrations. If we
combine the two reactants A and B, the forward reaction starts immediately,
but the formation of products allows the reverse process to get underway.
As the reaction proceeds, the rate of the forward reaction diminishes while
that of the reverse reaction increases. Eventually the two processes are
proceeding at the same rate, and the reaction is at equilibrium:
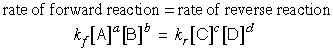
If we now change the composition of the system by adding some C or withdrawing
some A (thus changing their "active masses"), the reverse rate will exceed
the forward rate and a change in composition will occur until a new equilibrium
composition is achieved. This is referred to as the Law of Mass Action
and is thus essentially the statement that the equilibrium composition
of a reaction mixture can vary according to the quantities of components
that are present.
How can one tell when a reaction is in a state of
equilibrium? Clearly, if we observe some change taking place a change
in color, the emission of gas bubbles, the appearance of a precipitate,
or the release of heat, we know the reaction is not at equilibrium.
However, the absence of any sign of change does not by itself establish
that the reaction is at equilibrium, which is defined above as the lack
of any tendency for change to occur; "tendency" is not a property that
is directly observable! Consider, for example, the reaction representing
the synthesis of water from its elements:
2 H2 + O2 2 H2O
2 H2O
You can store the two gaseous reactants in the same
container indefinitely without any observable change occurring. But if
you create an electrical spark in the container or introduce a flame, bang!
After you pick yourself up off the floor and remove the shrapnel from what's
left of your body, you will know very well that the system was not initially
at equilibrium! It happens that this particular reaction has a tremendous
tendency to take place, but for reasons that we will discuss in a later
chapter, nothing can happen until we "set it off" in some way- in this
case by exposing the mixture to a flame, or (in a more gentle way) by introducing
a platinum wire, which acts as a catalyst. A reaction of this kind is said
to be highly favored thermodynamically, but inhibited kinetically.
The hydrogen iodide reaction, by contrast, is only moderately favored thermodynamically
(that's why it is incomplete), but its kinetics are reasonably facile.
It is almost always the case, however, that once
a reaction actually starts, it will continue on its own until it reaches
equilibrium, so if we can observe the change as it occurs and see it slow
down and stop, we can be reasonably certain that the system is in equilibrium.
This is by far the chemist's most common criterion.
There is one other experimental test for equilibrium
in a chemical reaction, although it is really only applicable to the kind
of reactions we described above as being reversible. As we shall see later,
the equilibrium state of a system is always sensitive to the temperature,
and often to the pressure, so any changes in these variables, however,
small, will temporarily disrupt the equilibrium, resulting in an observable
change in the composition of the system as it moves toward its new equilibrium
state. Similarly, addition or removal of one component of the reaction
will affect the amounts of all the others. If carrying out any of these
operations fails to produce an observable change, then it is likely that
the reaction is kinetically inhibited and that the system is not at equilibrium.