Electrochemical Cells
Everyone has used batteries or electrochemical cells from time-to-time, and many people often ask, just how do they work. The simple explanation is that they convert chemical energy to electrical energy. However, the actual chemistry involved to make this conversion happen can be quite complex. Technically, a battery is only created when two or more cells are connected together. When you purchase AA batteries, you are actually purchasing AA cells (read the package) and when you put them into some device and connect two or more of them in a single row or side-by-side, you are creating a battery. However, most people use the term battery improperly (tsk, tsk).
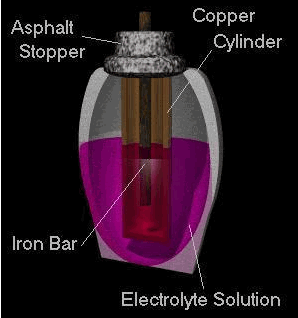
The ancient Greeks and Romans used to add electric eels to porous jars in pools of water. They would bathe in the water and receive mild electric shocks for therapeutic reasons or to empower them. Although the use is debatable, the fact that electricity could be generated is not. The first real evidence of a culture harnessing electrical power seems to be 250 B.C. near Baghdad. Clay jars have been excavated from this region, but these jars were unique because they contained a copper cylinder within which, an iron rod was suspended. The iron rod appears to be corroded due to the presence of an acidic solution (perhaps fruit juice or vinegar). It is know believed that these jars were they first (portable) cells; however, some dispute this claim as the jar don't seem to be functional (this is a key problem with historical relics; we can only infer their use).

There have been numerous scientists working with electricity throughout our past, but the first pivotal work is attributed to Luigi Galvani in 1790. He found that when different metals were attached to wires from an electrostatic generator and applied to a frog's leg, the muscles would twitch. He further discovered that the metals could induce muscle action without an external source of electricity. He thought this meant that animals contained an innate source of electricity and that fluids in the body allowed this to happen (he was sort of correct--animal tissues do generate their own electricity and that is how our muscles work).
Although most scientists accepted Galvani's ideas, Alessandro Volta did not. He believed that the source of the electricity was the metals and not the animal tissue (he is also sort of correct--animal tissues can generate electricity through a different process involving ion gates).

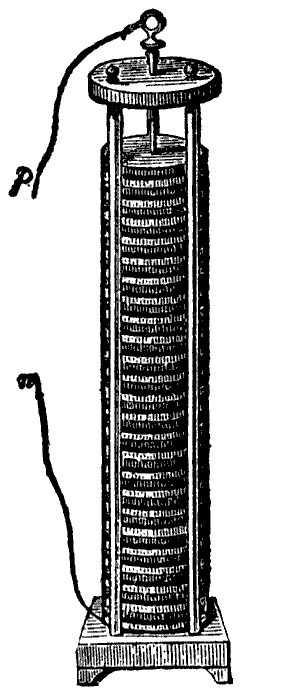
Volta constructed a device called an voltaic pile. It was constructed of numerous cells (voltaic cells) with alternating discs of copper and zinc separated by brine-soaked cardboard. It was the first device to consistently produce substantial and a steady supply of current. This was the first functioning battery.
To this day, electrical responses in tissues are called Galvanic responses and electrochemical cells are also known as
Voltaic cells. Voltaic cells are often called Galvanic cells as well.
The fundamental concept behind the functioning of an electrochemical cell is the ability of substances to transfer electrons in order to achieve higher stability. Therefore, this process is spontaneous and results in a loss of energy. This energy can then be harnessed and put to useful work; for example, your iPod.
This transfer of electrons is termed an oxidation/reduction reaction. Consider the following example:
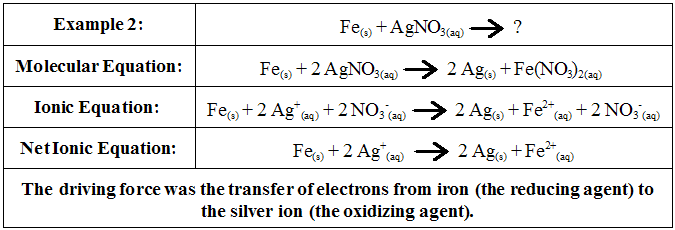
This example reveals a very unique type of ionic reaction; one in which there is simply a transfer of electrons from one species to another. These reactions appear simple but actually have profound effects in our environment. For example, rusting of metal is an example of such a reactions. These reactions are called redox reactions, or oxidation-reduction reactions and they have a number of similarities to acid-base reactions. Fundamentally, redox reactions are a family of reactions that are concerned with
the transfer of electrons between species. Like acid-base reactions, redox reactions are a matched set in that you don't have an oxidation reaction without a reduction reaction happening
at the same time. Each reaction by itself is called a "half-reaction", simply because we need two (2) half-reactions to form a whole reaction. The compound that loses an electron is said to be oxidized. This comes from the observation that materials combine with oxygen in varying amounts. For instance, an
iron bar oxidizes (combines with oxygen) to become rust. We say that the iron has oxidized. The substance which gains an electron is said to be reduced. Reduction was a term originally used in relation to iron ores. The iron in Fe2O3 has a 3+ charge, but the iron is FeO only has a 2+ charge. As a consequence of the the iron in FeO having one more electron, the ore would be lighter (per mole) or reduced in mass. This mass connection does not always hold true for other ores, but the term has been kept. Consider the following animation
(please note that it starts over):

Notice that the sodium atoms lose an electron (oxidation) while the chlorine atoms gain an electrons (reduction). Consider another example:
Fe(s) + Cu2+(aq) 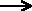 Fe2+(aq) + Cu(s)
Fe2+(aq) + Cu(s)
Fe(s) donates two electors (oxidation) to the Cu2+(aq) (reduction) to form Cu(s) . Since the Cu2+(aq) takes two electrons from the Fe(s) causing Fe(s) to be oxidized, Cu2+(aq)) is an oxidizing
agent. Likewise since Fe(s) forces Cu2+(aq) to accept two electrons, thus Fe(s) brings about reduction and is therefore a reducing agent. Try not to confuse the terms.
Remember:

The
success of this process relies on putting two substances with differing ability to attract/lose electrons together in a medium that allows easy movement of these free electrons. Metals are typically best for this since they have numerous electrons with are loosely held within a collective or have strong nuclei that attract other electrons. For example, when copper and silver are put together in a salt solution, silver has a stronger potential to remove electrons than copper and hence, copper loses electrons to silver. These exchanges can be written separately in their individual half-cell reactions or combined in a complete ionic reaction.
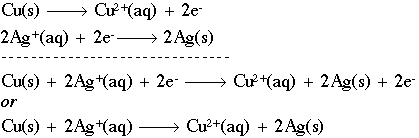
An electrochemical cell can be made using these half-cell reactions by placing each piece of metal or electrode in contact with an electrolyte (a conducting solution of ions). Current flows through the electrodes via the movement of electrons. The half-cells are connected by a cell separator that allows ions to move between the half-cells but prevents mixing of the electrolytes. The separator can consist of a tube or salt bridge of ions plugged at both ends with glass wool. Consider an electrochemical cell using copper and zinc electrodes.
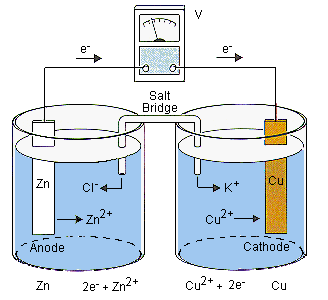
Here, copper has a stronger desire for electrons (reduction so that electrode is called the cathode because its negative charge will attract positive ions which are called cations) while zinc can more easily lose electrons (oxidation = anode). Electrons flow from the copper electrode to the copper ions bathing it. This causes electrons to flow from the zinc electrode to the copper electrode to replace the electrons that have been lost. This means the zinc electrode will be creating positive zinc ions and releasing them into the solution bathing it. This movement of electrons can be used to create other forms of energy such as sound in your iPod. This reaction would soon come to a screeching halt once all the copper ions were used up and the zinc ions saturate the zinc cell. This is where the salt bridge comes in to play. The salt bridge contains a salt that will remain soluble when it combines with the ions present in each beaker. In the example above, KCl is used and the increase in positive zinc ions in the zinc cell draws the negative chloride ions out of the zinc cell. At the same time, the dramatic reduction in positive copper ions in the copper cells causes that cell to become more and more negative which draws potassium ions out of the salt bridge. This reduces charge build up within each cells which means the reactive ions (copper and zinc) will remain free enough to engage the electrodes. Hence, the reaction will continue. Consider a more detailed version of this set up (the beakers are reversed).
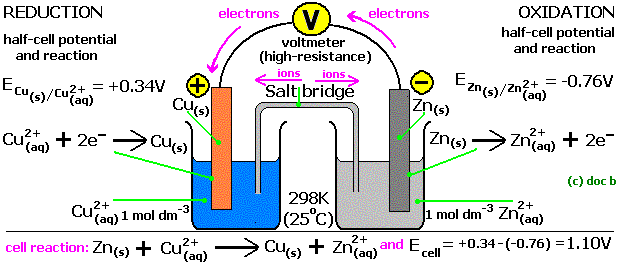
Note that in cells or batteries, the positive marking on the cell (look at an AA cell or car battery) is actually the cathode and receives the electrons, which means it is in fact negatively charged (there is more to this story but it is not needed here--suffice to say, this is not a mistake and there is a good reason for this quirk).
Cells we purchase (often mistaken for batteries) are called primary cells because they are irreversible. During use, reactants are converted to products, and when the reactants are used up, the cell "dead." Typically, these inexpensive cells are really a Leclanche dry cell. The body of the battery is made of zinc, which acts as the anode. A carbon rod in the center of the cell serves as the cathode. It is surrounded by a moist paste of graphite powder (carbon), manganese dioxide (MnO2), and ammonium chloride (NH4Cl). The anode reaction is the oxidation of the zinc cylinder to zinc ions. The cathode reaction involves the reduction of manganese dioxide. A simplified version of the overall reaction is
Zn + 2 MnO2 + H2O  Zn2+ + Mn2O3 + 2OH-;
Zn2+ + Mn2O3 + 2OH-;
Alkaline cells are similar, except that the zinc case is porous and the paste around the carbon cathode is moist manganese dioxide and potassium hydroxide. These are more expensive than ordinary zinc-carbon cells, but they maintain a high voltage for longer periods.
The lead-acid storage battery used in automobiles is a secondary battery; it is rechargeable using the alternator or by an external battery charger. The anode consists of porous lead plates in contact with a sulfuric acid (H2SO4) solution. The cathode consists of lead dioxide (PbO2) plates, also in sulfuric acid. Electrons flow from the lead plates to the lead oxide plates. As lead (Pb) loses electrons, it forms lead ions (Pb2+) that react with sulfate ions (SO42-) in solution to form insoluble lead sulfate (PbSO4). When PbO2 gains electrons during a recharge, it too reacts with SO42- ions in solution to form solid PbSO4. The cell reaction is
Pb + PbO2 + 4 H+ + 2 SO42-  2 PbSO4 + 2 H2O
2 PbSO4 + 2 H2O
and proceeds from left to right when the battery is discharging and from right to left when charging. The rechargeable nickel-cadmium (Ni-Cad) batteries are used in a variety of cordless appliances such as telephones, battery operated tools, and portable computers. During discharge, cadmium metal (Cd) acts as the anode, and nickel dioxide (NiO2) as the cathode. Both metals form insoluble hydroxides due to the presence of the potassium hydroxide electrolyte. The cell reaction during discharge is
NiO2 + Cd + 2 H2O  Ni(OH)2 + Cd(OH)2
Ni(OH)2 + Cd(OH)2
The reaction is reversed during charging.
Electrochemical Cell Potential
Electrical potential is created through contact between 2 dissimilar metals or conductors and this potential is a measure of the energy per unit charge due to the redox reaction between these conductors. If we tabulate the oxidation and reduction potentials of all available electrodes, then we could predict the cell potentials
of any combination. The electrode potential cannot be determined in isolation and the electrode potential depends upon the concentrations of the substances, the temperature, and the pressure in the case of a gas electrode.
Thus, to make a versatile table, the electrical potential of a conductor is measured in comparison to a standard hydrogen electrode which is given an arbitrary electric potential of 0 volts. Also, the values are standardized by using standard thermodynamic conditions for the measurement of the potentials. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 100 kPa and a standard temperature which is usually 25°C. The standard cell potential is denoted by a degree sign as a superscript.
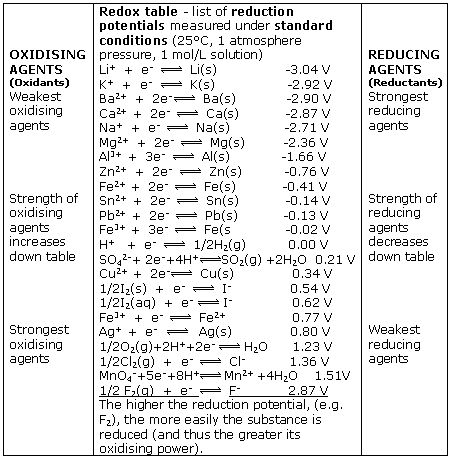
Working out the mechanics and overall equations for any 2 electrode combination is no easy feat, but the benefits have been tremendous and permeate all aspects of our modern culture.
For example, consider the hydrogen-oxygen fuel cell used by the space shuttle. The electrodes are hollow carbon tubes impregnated with a catalyst and the electrolyte is hot, concentrated KOH. Hydrogen gas is pumped to the anode to give:
H2 + 2 OH-  2 H2O + 2 e-
2 H2O + 2 e-
At the same time, oxygen gas is pumped to the cathode to give:
O2+ 2 H2O + 4 e-  4 OH-
4 OH-
The net result is:
2 H2(g) + O2(g)  2 H2O(l)
2 H2O(l)
The water produced generates immense amounts of electricity to run the shuttle and supplies the astronauts with
drinking water. If the reaction is linked to a solar panel it can be reversed to provide the reactants to repeat the
electrochemical process to again supply energy when needed and hence this could represent an alternative fuel
for our society that would be clean and self-perpetuating
(this recharge cycle has some large obstacles to overcome yet).
Consider another example. Unsaturated fats (long chain carbon molecules with double bonds present) are oxidized by oxygen in the air
which causes them to break down or spoil. Oxygen removes a hydrogen from the fat molecule to create free radicals which are atoms or molecules with
unpaired electrons. This is an important factor in the freshness of potato chips; thus, chips would spoil when exposed to air. To combat this, antioxidants are added to the chips (and other foods). Two of the most popular antioxidants are BHT (butylated hydroxytoluene) and BHA (butylated hydroxyanisole) as they specifically prevent the oxidation of fats.
Just to note: we consume on average 0.1 mg/kg of body weight of these each day. Attests show that doses 500 times greater cause your liver to enlarge and they are linked to reproductive difficulties
in animals. Free radicals are thought to cause many of the effects of aging: winkling of skin, DNA damage and failure and protein degradation (causes stiffness and loss of flexibility and enzyme malfunction). Vitamin E is a natural antioxidant and a deficiency of it produces many of the effects of aging.
Electrolytic Cells
What would happen if electricity was added to a galvanic or voltaic cell. Well, the electrochemical cell becomes an electrochemical cell producing electricity to an electrolytic cells consuming electricity. But why would we want to do that. Simply put, to build electrodes that we could not other build so easily.
The structural difference between the two types of cells is that a galvanic cell must use dissimilar metals which are separated, except for ions, to produce a charge; whereas, an electrolytic cell has both anode and cathode suspended in the same solution and is driven by an external electrical charge. Also an electrolytic cell may use the same metal for cathode and anode.
The electrolytic cell simply runs in the opposite direction (reduction still = gain of electrons and oxidation still = loss of electrons, but where this is happening changes). The result though, is the deposition of desirable metal ions onto one of the electrodes. This is how electroplating is conducted to cover copper with gold or to prepare aluminum siding which allows us to use a cheaper or harder metal to become coated with a more valuable metal and as result produce a longer lasting and more appealing end result. Consider the following example of how a cheap metal such as steal could be given a coating of silver to increase it value.
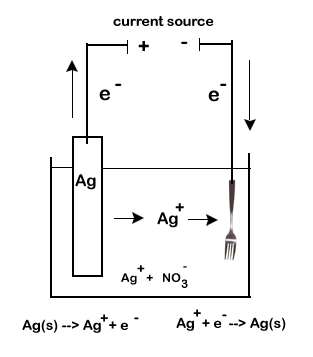
References
http://www.ihatebatteries.com/BatteryHistory/
http://skepticworld.com/ancient-artifacts/baghdad-battery.asp
http://inventors.about.com/library/inventors/bl_Galvani.htm
http://www.bo.infn.it/galvani/galvani.gif
http://www.thevillapassalacqua.com/images/uploads/volta.jpg
http://inventors.about.com/library/inventors/bl_Alessandro_Volta.htm
http://etc.usf.edu/clipart/4200/4286/voltaic-pile_1_lg.gif
http://www.shodor.org/unchem/advanced/redox/index.html
http://cslamerica.org/Image3.gif
http://www.docbrown.info/page07/addhoc07/cell1.gif
http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/electrode.html
http://hsc.csu.edu.au/chemistry/options/industrial/2763/images/ch954_2.gif
http://www.answers.com/topic/electrolytic-cell
http://www.saskschools.ca/curr_content/chem30_05/graphics/6_graphics/fork.gif

